Direct naar Forum

|
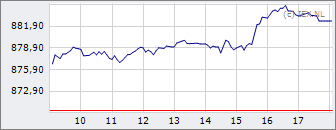
AEX
882,63
+12,36
+1,42%
26 apr
|
|
|
|

|
Germany40^ |
18.177,90
|
+1,45%
|
|

|
BEL 20 |
3.874,87
|
+0,44%
|
|

|
Europe50^ |
5.011,70
|
+0,10%
|
|

|
US30^ |
38.211,61
|
0,00%
|
|

|
Nasd100^ |
17.698,09
|
0,00%
|
|

|
US500^ |
5.095,29
|
0,00%
|
|

|
Japan225^ |
38.345,55
|
0,00%
|
|

|
Gold spot |
2.337,95
|
0,00%
|
|

|
EUR/USD |
1,0693
|
0,00%
|
|

|
WTI |
83,64
|
0,00%
|
|
#/^ Index indications calculated real time, zie
disclaimer