Direct naar Forum

|
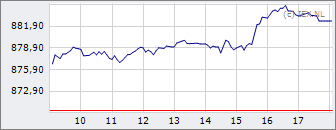
AEX
900,06
+8,93
+1,00%
07 mei
|
|
|
|

|
Germany40^ |
18.444,40
|
+0,08%
|
|

|
BEL 20 |
3.996,52
|
+1,72%
|
|

|
Europe50^ |
5.011,28
|
-0,10%
|
|

|
US30^ |
38.854,63
|
0,00%
|
|

|
Nasd100^ |
18.096,28
|
0,00%
|
|

|
US500^ |
5.188,03
|
0,00%
|
|

|
Japan225^ |
38.711,22
|
0,00%
|
|

|
Gold spot |
2.310,82
|
-0,14%
|
|

|
EUR/USD |
1,0738
|
-0,17%
|
|

|
WTI |
78,46
|
0,00%
|
|
#/^ Index indications calculated real time, zie
disclaimer