Direct naar Forum

|
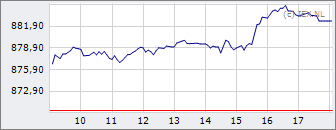
AEX
860,01
-5,35
-0,62%
18:05
|
|
|
|

|
Germany40^ |
17.711,30
|
-0,71%
|
|

|
BEL 20 |
3.827,75
|
+0,03%
|
|

|
Europe50^ |
4.909,10
|
-0,56%
|
|

|
US30^ |
37.931,95
|
-0,21%
|
|

|
Nasd100^ |
17.087,68
|
-2,63%
|
|

|
US500^ |
4.973,97
|
-1,49%
|
|

|
Japan225^ |
37.188,48
|
-2,14%
|
|

|
Gold spot |
2.395,14
|
+0,66%
|
|

|
EUR/USD |
1,0653
|
+0,09%
|
|

|
WTI |
82,41
|
+0,40%
|
|
#/^ Index indications calculated real time, zie
disclaimer
-
Premium


-
Premium


-
Premium


-
Premium


-
Premium

